

As a cleansing agent for domestic purposes like washing clothes.Some common applications of sodium carbonate include: It is one of the few metal carbonates that is soluble in water. Na 2 CO 3 + 10 H 2 O ⟶ Na 2 CO 3 ⋅ 10 H 2 O Soda ash is dissolved in water and crystallized to get washing soda. Sodium carbonate decahydrate (Na 2CO 3♱0H 2O), also known as washing soda, is the most common hydrate of sodium carbonate containing 10 molecules of water of crystallization. with 2.5 units of water per sodium carbonate unit ("pentahemihydrate"). In dry air the decahydrate and heptahydrate lose water to give the monohydrate. The decahydrate is formed from water solutions crystallizing in the temperature range −2.1 to +32.0 ☌, the heptahydrate in the narrow range 32.0 to 35.4 ☌ and above this temperature the monohydrate forms. It is also formed when sodium hydrogencarbonate is heated (calcined) e.g. anhydrous sodium carbonate ( natrite), also known as calcined soda, is formed by heating the hydrates.sodium carbonate monohydrate ( thermonatrite), Na 2CO 3.sodium carbonate heptahydrate (not known in mineral form), Na 2CO 3♷H 2O.sodium carbonate decahydrate ( natron), Na 2CO 3♱0H 2O, which readily effloresces to form the monohydrate.Sodium carbonate is obtained as three hydrates and as the anhydrous salt: It is produced in large quantities from sodium chloride and limestone by the Solvay process, as well as by carbonating sodium hydroxide which is made using the Chlor-alkali process. Because the ashes of these sodium-rich plants were noticeably different from ashes of wood (once used to produce potash), sodium carbonate became known as "soda ash". Historically, it was extracted from the ashes of plants grown in sodium-rich soils. All forms are white, odourless, water-soluble salts that yield alkaline solutions in water. Sodium carbonate (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na 2CO 3 and its various hydrates. “Use of Silver Carbonate in the Wittig Reaction.” The Journal of Organic Chemistry 78.23 (2013): 12224–12228. "Thermal Decomposition of Silver Carbonate: Phenomenology and Physicogeometrical Kinetics".

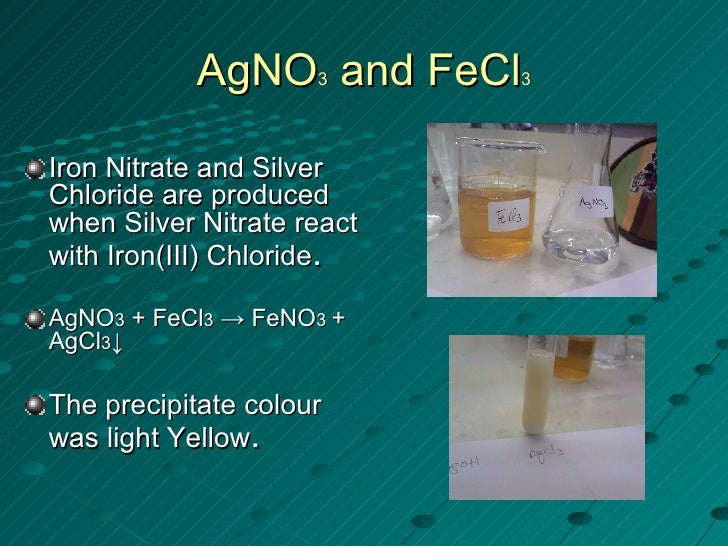
Solubilities of Inorganic and Organic Compounds (2nd ed.). CRC Handbook of Chemistry and Physics (90th ed.). As a base, it has been used in the Wittig reaction. It is also employed to convert alkyl bromides into alcohols. In the Fétizon oxidation, silver carbonate on celite serves as an oxidising agent to form keto alcohols from diols. Silver carbonate is used as a reagent in organic synthesis such as the Koenigs-Knorr reaction. 2 AgNO 3 ( aq ) + Na 2 CO 3 ( aq ) ⟶ Ag 2 CO 3 ( s ) + 2 NaNO 3 ( aq ) Silver carbonate can be prepared by combining aqueous solutions of sodium carbonate with a deficiency of silver nitrate. It is poorly soluble in water, like most transition metal carbonates. This salt is yellow but typical samples are grayish due to the presence of elemental silver. Silver carbonate is the chemical compound with the formula Ag 2CO 3.


 0 kommentar(er)
0 kommentar(er)
